Dissociation of H2co3 in Water Equation
Carbon dioxide dissolves in water to form carbonic acid. H X 2 C O X 3 H X 2 O C O X 2.

Carbonic Acid Formation Structure Chemical Equation Video Lesson Transcript Study Com
All the reactions in this series are two-way in other words the.
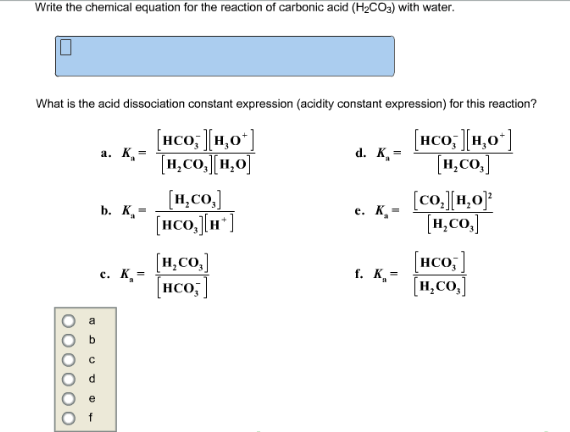
. Write the chemical equation for the reaction of carbonic acid H2CO3 with water. Chemistry questions and answers. The balanced equation for the formation of carbonic acid is CO2 H2O H2CO2 H HCO3-.
Carbonic acid forms from the dissolution of water or H 2 O in carbon dioxide or CO. Carbonic acid is often descri. And then you have to take into account another important equilibrium many time ignored.
Carbonated water is a solution of carbonic acid H2CO3. The chemical equation is written below. The equations are as follows.
Carbonates is the control which dissolved carbon dioxide has on water pH and buffering. Solutions of carbon dioxide in water contain small amounts of this compound. The equation for the dissociation of acetic acid for example is CH3CO2H H2O CH3CO2 H3O.
In this instance water acts as a base. The first dissociation step is. Where the equilibrium conditions are quantified by the dissociation or acidity constants.
C O X 2. Its chemical formula can also be written as OCOH2 since there exists one carbon-oxygen double bondin this compound. The concentration of undissociated H2CO3 cannot be measured in solution because it rapidly dissociates into CO2 and H2O or to H and HCO3-.
Both of these acids are weak acids. Write the chemical equation for the reaction of carbonic acid H2CO3 with water. Yes but in fact this is an equilibrium.
What is the acid dissociation constant expression acidity. What is the acid dissociation constant expression acidity constant expression for. It is formed in small amounts when its anhydride carbon dioxide CO2 dissolves in water.
H CO H HCO K 2 3 3 1 99 and 3 HCO H CO K 3 2 2 910 Finally the dissociation of water. H2O CO2H2CO3H and HCO3-another H and CO3 with a charge of -2. CO2 H2O H2CO3 The predominant species are simply loosely hydrated CO2 molecules.
The chemical equation is given below. Carbonic acid is a carbon-containing compound which has the chemical formula H2CO3. Write equations to show the ionization of each acid when placed into water.
Dissociation constants for carbonic acid CO2 H2O NH HCO 2 K 1 1 HCO3 NH CO 3 2 K 2 2 The dissociation constants are defined by K1 H HCO 3 CO2 3 K2 H CO 3 2 HCO 3 4 where i. Estimate the thermodynamic equilibrium constanst K for this reaction delta Gf values. Christian on 11 Jun 2018.
H2BO3- H HBO3-2 Ka2 18. PK 1 dissociation 1 log β 2 log β 1 association In non-biological solutions. In this case the water molecule acts as an.
First let us start with the first dissociation equation resulting in the release of bicarbonate ions along with protons. Carbonic acid dissociates into bicarbonate and carbonate according to the following. 0 comments Carbon dioxide C O 2 dissolves in water and some of the dissolved C O 2 forms carbonic acid H 2 C O 3 a q.
The hydration equilibrium constant at 25 C is called K h which in the case of carbonic acid is H 2 CO 3CO. H3BO3 H H2BO3- Ka1 73 x 10-10. Sodium carbonate is an ionic compound and its dissociation will lead to the formation of its respective ions.
The second dissociation step is.
What Is Carbonic Acid Ionization Quora

How To Balance H2co3 H2o Co2 Decomposition Of Carbonic Acid Youtube
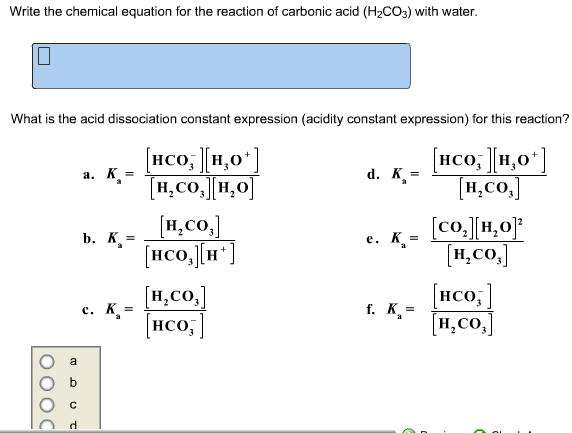
Solved Write The Chemical Equation For The Reaction Of Chegg Com

Solved Write The Chemical Equation For The Reaction Of Chegg Com
No comments for "Dissociation of H2co3 in Water Equation"
Post a Comment